Citric Acid
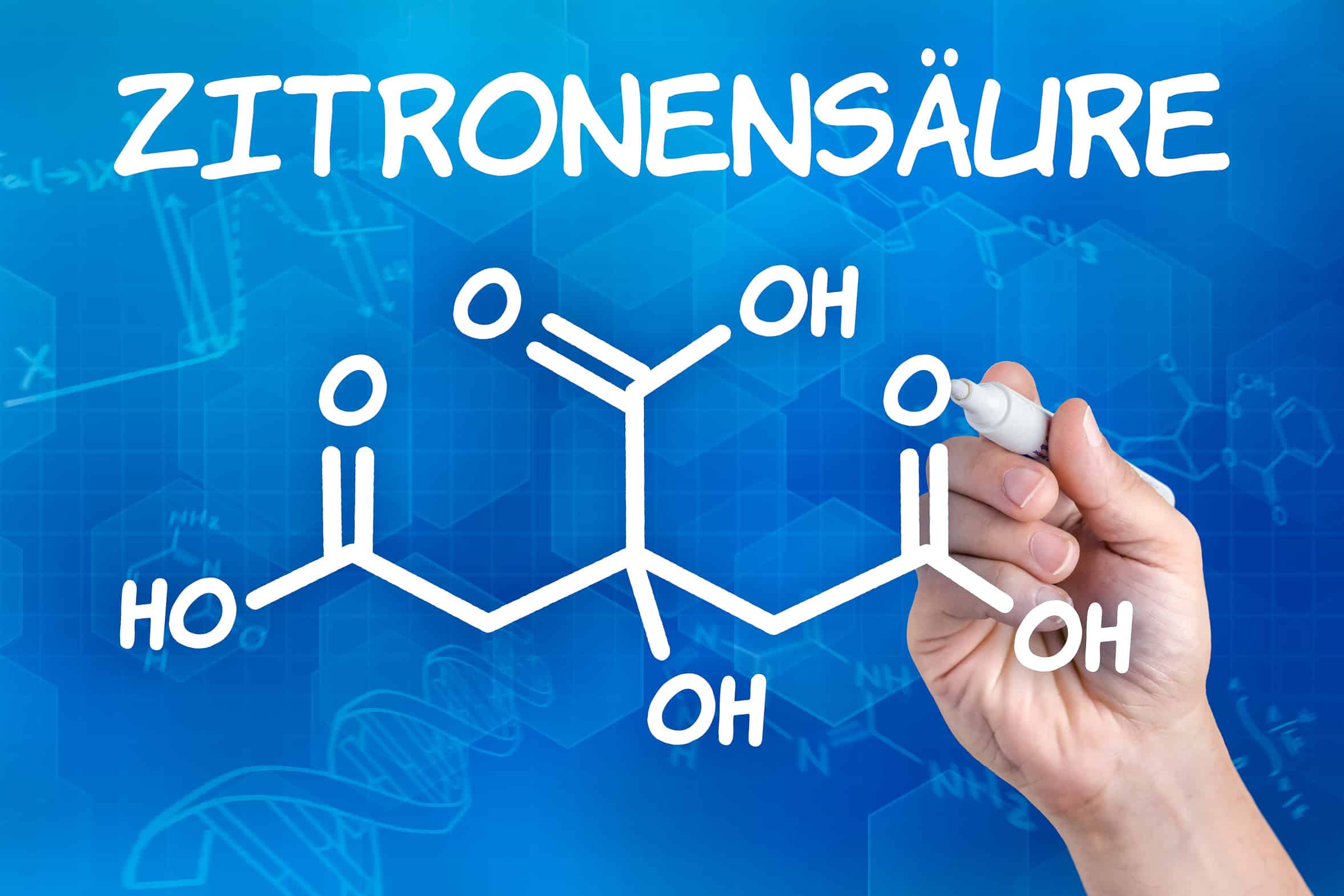
It is the most widespread acid in the plant kingdom. Lemon juice contains between 5% and 7% of this acid, but it is also found in apples, pears, sour cherries, etc., even in milk. It also occurs in every organism as a metabolic product.
Citric acid and citrates are chemical compounds that occur naturally and are used in many different applications, including the cosmetics industry.
The citrate cycle
Citric acid is an intermediate product of the citrate cycle is. Process is the key role in the carbohydrate – and fatty acid – metabolism of all oxygen-consuming organisms. Humans are also included in this process. In addition, the cycle provides the basic molecular structures for the formation of most amino acids.
The citrate cycle is a biochemical process that occurs in all aerobic organisms and enables the conversion of nutrients such as glucose, fatty acids, and amino acids into energy by oxidative phosphorylation.
The citrate cycle begins with the condensation of acetyl-CoA with oxaloacetate to citrate, producing citric acid as an intermediate. Citric acid is then converted to several other tricarboxylic acids, including succinyl-CoA, fumarate, malate, and oxaloacetate, through a sequence of enzymatic reactions. This produces energy in the form of reduced nicotinamide adenine dinucleotide (NADH) and reduced flavin adenine dinucleotide (FADH2), which are later used in the respiratory chain to produce ATP.
The citrate cycle is an important process for the body’s energy balance, and dysfunction in this process can lead to various metabolic diseases. For example, under-functioning of the citric acid cycle is associated with various neurological disorders and muscle weakness, while over-functioning is associated with certain cancers.
Overall, the acid is an important component of the citrate cycle, which plays a key role in the body’s energy balance and regulates many important biological processes.
Physicochemical Properties
Citric acid is an organic acid that exists in a water-soluble form. It has an acidic taste and is hygroscopic, meaning that it can absorb moisture from the air. In addition to the anhydrous version, there is also one with water, citric acid monohydrate (C6H8O7 + H2O). For every molecule of citric acid, there is one molecule of water of crystallization.
The compound is highly soluble in water and has a pH of about 2.2 to 2.5, but it is not soluble in oils and fats. Citric acid is also soluble in alcohol, but only to a limited extent in ethanol, as it can flocculate at higher concentrations.
Citric acid is a weak organic acid found in citrus fruits such as lemons, limes, and oranges. It is used in the cosmetics industry as an ingredient in various products, including skin care products, shampoos and hair conditioners, soaps, body washes and other cleansing products.
Citrates are salts of citric acid and are often used as buffering agents and pH regulators in cosmetic formulations. They can also be used as preservatives to extend the shelf life of cosmetic products.
Sodium citrate and potassium citrate are examples of citrates commonly used in the cosmetics industry. They can also be used in other industries such as food and beverage and pharmaceuticals.
Possible applications of citric acid
The applications of citric acid are very widespread. The acid is used in many household cleaners due to its acidic and water softening properties. Here are some examples of how the acid is used in household cleaners:
Descalers: the acid is commonly used in descalers for kitchen and bathroom surfaces such as sinks, shower stalls, faucets, tiles, and toilets. The acid helps remove mineral deposits and limescale that build up on surfaces over time.
Dishwashing detergent: citric acid is also used in dishwashing detergents to remove stubborn stains and discoloration from dishes, glasses, and silverware. The acid helps dissolve grease deposits and prevent water spots from forming on surfaces.
All-Purpose Cleaners: the acid is also used in all-purpose cleaners to remove dirt, stains, and discoloration from various surfaces such as floors, countertops and walls. The acid helps dissolve grease and oil and make surfaces shiny.
Detergent: can also be used as an ingredient in laundry detergents to remove stubborn stains on fabrics. The acid helps to dissolve dirt and oil and make clothes whiter again.
However, it is important to note that citric acid can also be aggressive in higher concentrations and can cause irritation when it comes into contact with skin and eyes. Therefore, products containing citric acid should always be used according to label instructions and kept away from children and pets.
Citric acid in cosmetics
There are very many other uses of the salts of citric acid. It is widely used in the cosmetics industry as an ingredient in various products, including skin care products, shampoos and hair conditioners, soaps, body washes and other cleaning products.
The acid has a mild exfoliating effect and can help remove dead skin cells to promote a smoother and more radiant complexion. Thus, for some time, hydroxycarboxylic acid (AHA) has been used as a chemical exfoliant for skin renewal.
Citric acid is also often used as a pH regulator, as it helps stabilize and maintain the pH of cosmetic formulations. A balanced pH is important for skin health and can help minimize skin irritation and other adverse reactions.
There are two reasons why the acid is so often added to skin creams. First, its weakly acidic nature makes it effective against fungal infections, and second, it binds calcium – and other heavy metal ions by complexing them. Otherwise, the calcium ions fix the dirt on the skin and the sparingly soluble calcium citrate thus binds the dirt to the skin surface. The heavy metals in turn promote autoxidation of some components of the cream and therefore it is important to bind and inactivate these heavy metals.
Fact Sheet:
INCI: Citric Acid
CAS No.: 77 -92- 9 (anhydrous); 5949- 29 -1 (monohydrate).
Other designations: E330, 2 – hydroxypropane – 1,2,3 – tricarboxylic acid.
Molecular formula: C6H8O7
Boiling point: decomposition starts at 175°.
Properties: colorless, odorless solid
Solubility: soluble in water
An important additive in cosmetics
Nowadays, citric acid is found almost everywhere and it is impossible to imagine our daily used products without it. For almost officially 300 years now, theacid has been used for a great many things. As a physiologically effective acid, it is very important for our organism and absolutely skin-friendly. Due to its buffering effect, it is hard to imagine the cosmetics industry without it. We at Cosmacon will be happy to advise you in choosing the right pH value for your products!
Costing services cosmetics is very simple at Cosmacon: After your enquiry, you will receive a complete overview of the maximum costs within 24 hours. Once we have signed a non-disclosure agreement, we will make you a fixed price offer for your desired products.
Sources:
Microbial citric acid: Production, properties, application, and future perspectives.; Behera, B. C., Mishra, R., & Mohapatra, S. (2021). Food Frontiers, 2(1), 62-76.
Citric acid from Aspergillus niger: a comprehensive overview.; Crit Rev Microbiol. 2020 Nov;46(6):727-749.
Dual Effects of Alpha-Hydroxy Acids on the Skin.; Molecules. 2018 Apr 10;23(4):863.
